About US
About KINDA Pharma
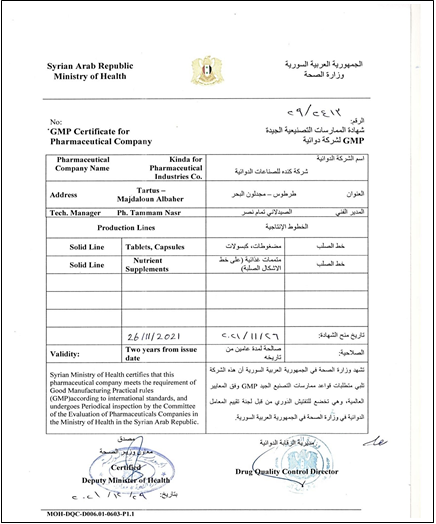
Kinda for Pharmaceutical Industries Co. is a privately owned joint-stock company established on 11/09/2014 in Tartous, Syria. The company Based in Majdaloun Al-Bahr village, Tartous, Syria. It specializes in the manufacturing of various pharmaceutical human medicines, including solid, semi-solid, and liquid forms, as well as ampoules, vials, drops and serums. Kinda Pharma also engages in import, export, and marketing activities (domestic and international), and invests towards achieving its objectives.
Currently, the company's operations are limited to solid production only, with gradual expansion planned for future inclusion of all activities. The solid production line was licensed on 30/01/2019 for manufacturing solid forms (tablets and capsules) and nutritional supplements
Kinda Vision
Kinda Pharma endeavors to pioneer the pharmaceutical industry through innovation and excellence, inspiring others to elevate healthcare on a global scale.
Kinda Mission
Kinda Pharma for Pharmaceutical Industries is dedicated to producing high-quality pharmaceuticals to meet global treatment needs and support healthcare professionals in their efforts.

Quality System Management at Kinda Pharma Co
Our Quality Management System is founded on the principles of Good Manufacturing Practices (GMP) and compliance with the ISO 9001:2015 standard. These standards serve as the framework for our commitment to delivering superior pharmaceutical products while ensuring continuous improvement in our operations.
Key aspects of our Quality Management System include:
- Adherence to recognized quality standards and regulatory requirements.
- Meeting the expectations of our customers and other relevant stakeholders.
- Continuous monitoring of process performance to ensure adherence to standards and integration across operations.
- Continuous training and development programs for our employees to enhance their skills and knowledge.
The management team takes responsibility for implementing and enforcing our quality policy in collaboration with all departments. To ensure the effectiveness of our Quality Management System, internal audit processes are conducted at least once annually to evaluate its performance and identify areas for improvement.

As part of our Quality Management objectives for 2024, we aim to achieve additional ISO certifications complementing our Quality Management System by mid-year. These certifications include ISO 45001:2018 for Occupational Health and Safety Management, as well as ISO 14001:2015 for Environmental Management. This endeavor underscores our commitment to ensuring comprehensive standards across health, safety, and environmental practices within our operations.
Basic training arrangements
- Maintaining regular employee education is done through various seminars and training courses, attracting distinct resources and collaborating with local and international expertise
- Training and education is carried out by a person with training qualifications and experience to qualify employees of the company that contributes to (GMP-ISO:9001) Corporate development in all fields that are required
- The training needs are determined by the department heads to suit the developmental needs of both the individual and the company by:
- The managers of departments shall identify the immediate requirements for individual training of members and staff by functional program or job description
- Further development training is determined by department managers as the role of the individual develops, systems and procedures are changed or as a result of changes in existing legislation affecting the functioning of the work
- Kinda Pharmaceutical Industries is also interested in training and developing its employees. It is committed to providing support to young people who aspire to achieve their professional dreams as part of our societal responsibility. Therefore, we offer specialized internal training programs in cooperation with government and private agencies and charitable organizations, with the aim of enabling students, graduates and job seekers to obtain the necessary skills and guidance necessary to build a professional future.
We believe in the importance of supporting young talent and motivating them to achieve their full potential
Through our partnerships with private entities, government and charitable associations, we work hard to enhance educational and training opportunities for young people in our communities. Our belief in the power of education and training drives us to provide distinguished programs that contribute to building a distinguished and creative future generation. Our special training programs include professional guidance and enhancing participants’ scientific and practical knowledge.” With the aim of preparing them for the labor market with confidence and competence.
Our Quality Management System is founded on the principles of Good Manufacturing Practices (GMP) and compliance with the ISO 9001:2015 standard.
Kinda Phamra
we are committed to ensuring comprehensive standards across health, safety, and environmental practices within our operations.